|
Listen to this article
|

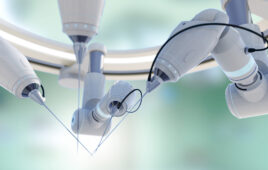
While Johnson & Johnson has mostly kept its Ottava surgical robot under wraps, it said the system offers surgical teams the freedom and flexibility to adapt to clinical workflows and individualize patient needs. | Source: Adobe Stock
Johnson & Johnson MedTech announced today that the U.S. Food and Drug Administration granted its Ottava surgical robot investigational device exemption, or IDE.
The regulatory nod comes just under a month after the company said it submitted Ottava for IDE. It allows Johnson & Johnson MedTech to begin a clinical trial for the robotic platform at U.S. sites. The company is now preparing clinical trial sites to receive Ottava systems, enroll patients, and begin surgical cases.
Johnson & Johnson first shared details on the Ottava surgical robotic platform about four years ago. However, since then, the company has remained quiet on the topic, though. In October 2021, J&J pushed back the platform’s development timeline by about two years due to multiple factors.
The initial unveiling of Ottava highlighted a six-armed approach for the robot. A November 2023 update shed more light on Ottava and some changes in its look over the past three years.
The company said Ottava incorporates four robotic arms into a standard-size surgical table. Its unified architecture allows for an “invisible design,” according to J&J. The robotic arms are available when needed and are stowed beneath the surgical table when not.
Johnson & Johnson MedTech said the design removes barriers to movement and collaboration in robotic operating rooms. It also offers surgical teams the freedom and flexibility to adapt to clinical workflows and individualize patient needs.
“We are bringing the best of J&J MedTech’s surgery expertise to the Ottava system and taking a holistic view of the science of surgery to enable new experiences across all surgical modalities in service of patients around the world,” said Hani Abouhalka, group chair for surgery at Johnson & Johnson MedTech. “Meeting this milestone brings us a step closer to delivering on our promise to make technology more human, care more adaptive, and people more connected so that surgery works better for everyone.”
Johnson & Johnson designs Ottava for versatility
Johnson & Johnson said it designed Ottava to set a new standard for the modern operating room and transform the surgical experience. The system’s unique architecture combines with J&J’s digital ecosystem to deliver versatility in meeting patient and surgeon needs.
With the four low-profile robotic arms, the company designed Ottava to support robotic, laparoscopic, hybrid, and open surgery with more working space for clinical teams. The system’s architecture supports features like “twin motion” in which the table and robotic arms move together. This aids in intraoperative repositioning and multi-quadrant access without re-docking.
Ottava also exclusively features trusted Ethicon instrumentation designed for performance and precision. J&J said this supports a more consistent experience between traditional laparoscopic and robotic procedures.
The company said its Polyphonic digital ecosystem can connect the portfolio across surgical technologies, robotics, and software while also using its scale.
In the future, J&J said it expects Polyphonic to empower Ottava with data and advanced insights. This would support clinical decision-making, learning and collaboration.
“We are excited about reaching this important milestone and progressing our differentiated general surgery robotic platform for the benefit of patients and surgeons,” said Rocco De Bernardis, president of Ottava at Johnson & Johnson MedTech. “With approval to move to clinical investigation, our teams are focused on training clinical trial investigators and teams as they enroll patients and prepare for cases.”
Editor’s note: This article was syndicated from The Robot Report sibling site MassDevice.





Lots of talk and dream. Where is the mock up? All hype so far.